Implementation of the RoHS Directive
Information about the implementation of the RoHS Directive, including the exemption procedure, timeframe and assessment studies.
Exemptions Procedure
The RoHS Directive allows for exemptions from its restrictions, under certain conditions defined in article 5(1), adapting the Annexes to scientific and technical progress.
Exemptions are limited in time and reassessed on a regular basis, taking into account
- the availability, practicability and reliability of substitutes
- the environmental, health and consumer safety impacts of substitution
- the socioeconomic impact of substitution
- any potential adverse impacts on innovation
Industry regularly applies for the renewal of exemptions or for additional applications to be exempted from the Directive's requirements. Each request must be evaluated, and when appropriate, an exemption is granted.
Exemptions Timeframe
A decision on a RoHS exemption currently takes 18 to 24 months from the application date. Priority is given to older applications. Existing exemptions for which a renewal request has been submitted remain valid until a decision is taken by the Commission. This decision either indicates a new expiry date or, in case of rejection, grants a transition period of 12 to 18 months before the exemption expires. Exemptions for which no application for renewal was submitted in due time will expire on the date specified in Article 5 or in the relevant annex of the Directive.
After submitting a request for a new exemption, equipment must comply with the Directive to be placed on the Union market, until a decision granting a new exemption is adopted by the Commission.
Review
Review of RoHS 1
The aim of the RoHS recast was, among other things, to reduce administrative burdens and ensure coherency with newer policies and legislation. These cover, for example, chemicals and the new legal framework for marketing products in the European Union.
Free Sample Report Performance Quality Control
Download a sample report to keep control of your supply chain!
Featured Articles
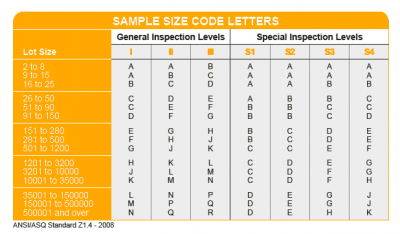 AQL Table | How to Read It
AQL Table | How to Read It TOP 10 Common Defects in Garments Quality Inspection
TOP 10 Common Defects in Garments Quality Inspection Product Packaging and Shipment Label requirements for Amazon FBA
Product Packaging and Shipment Label requirements for Amazon FBA What Is ASTM-F2413-18? Protective Footwear Standard
What Is ASTM-F2413-18? Protective Footwear Standard How to Conduct Third-Party Quality Control Inspections for Electric Scooters
How to Conduct Third-Party Quality Control Inspections for Electric Scooters SMETA Audit-What is SMETA Audit?
SMETA Audit-What is SMETA Audit? TESTCOO Supplier Verification/Certification Service SLCP, Higg FEM, GRS, GOTS
TESTCOO Supplier Verification/Certification Service SLCP, Higg FEM, GRS, GOTS Quality Control Inspection Company in China
Quality Control Inspection Company in China What is Quality Inspection? A Complete Guide
What is Quality Inspection? A Complete Guide Guidelines for Product Inspection in India
Guidelines for Product Inspection in India
Category
- Production Inspection Service
- Factory Audit
- Softline Inspection
- Hardline Inspection
- Electrics Inspection
- Certification
- Checklist
- Manufacturers
- Quality Assurance Basics
- Products Recall
- AQL
- Guidence and Standard
- News
- Supplier Management
- Amazon
- Protective Equipment
- e-commerce quality control
- Indian Manufacturing
- Soft Goods Quality Control
- Supply Chain Management
- Supply Chain Resilience
- E-Commerce Quality Control
- ISO 2859
- Supply Chain Optimization
- Garment Industry
- Higg Index



